is carbon dioxide polar Carbon dioxide polar co2 nonpolar science
Today, we will be discussing two fascinating topics related to bond polarity and carbon dioxide. Bond polarity is an essential concept in chemistry that helps us understand the nature of chemical reactions. On the other hand, carbon dioxide is a compound that plays a vital role in our daily lives. Let’s delve into these subjects to explore their significance and impact.
Bond Polarity
Bond polarity refers to the distribution of electron density between two atoms in a chemical bond. It is determined by the difference in electronegativity values of the elements involved in the bond. Electronegativity is a measure of an atom’s ability to attract electrons towards itself.
 In the image above, we can see a visual representation of bond polarity. This concept is fundamental in understanding the nature of chemical bonds, as it helps predict the behavior of molecules in various reactions.
In the image above, we can see a visual representation of bond polarity. This concept is fundamental in understanding the nature of chemical bonds, as it helps predict the behavior of molecules in various reactions.
One example of bond polarity is the covalent bond between hydrogen and oxygen atoms in a water molecule (H2O). Oxygen has a higher electronegativity than hydrogen, resulting in an uneven distribution of electron density. As a result, the oxygen atom becomes slightly negative (δ-) and the hydrogen atoms become slightly positive (δ+).
Bond polarity is vital in determining the physical and chemical properties of a compound. Polar molecules tend to have higher boiling points, as the positive and negative charges attract each other, requiring more energy to break the intermolecular forces. On the other hand, nonpolar molecules, such as hydrocarbons, do not possess dipole moments and have lower boiling points.
Carbon Dioxide: Polar or Nonpolar?
Now, let’s shift our focus to carbon dioxide (CO2). Carbon dioxide is a molecule composed of one carbon atom bonded to two oxygen atoms. To determine whether CO2 is polar or nonpolar, we need to consider its molecular geometry.
 The image shown above represents the molecular structure of carbon dioxide. As we can see, the carbon-oxygen bonds are linear, and the molecule possesses a symmetrical arrangement. Due to the equal electronegativity of carbon and oxygen atoms, the molecule’s dipole moments cancel out, resulting in a nonpolar molecule.
The image shown above represents the molecular structure of carbon dioxide. As we can see, the carbon-oxygen bonds are linear, and the molecule possesses a symmetrical arrangement. Due to the equal electronegativity of carbon and oxygen atoms, the molecule’s dipole moments cancel out, resulting in a nonpolar molecule.
Even though carbon dioxide is a nonpolar molecule, it plays a significant role in our environment. It is a greenhouse gas, meaning it contributes to the greenhouse effect and global warming. The accumulation of carbon dioxide in the atmosphere is primarily responsible for the increasing temperatures on Earth.
Carbon dioxide is also essential for photosynthesis, a process that occurs in plants and algae. During photosynthesis, carbon dioxide is converted into organic compounds with the help of sunlight. This process not only provides energy for plants but also acts as a crucial carbon cycle, regulating the levels of carbon dioxide in the atmosphere.
In conclusion, understanding bond polarity and the polarity of molecules like carbon dioxide is crucial for comprehending the behavior of chemical compounds and their impact on our environment. As we delve deeper into these subjects, we gain insights into the intricate world of chemistry and its relevance to our daily lives.
If you are searching about Bond Polarity | CK-12 Foundation you’ve visit to the right place. We have 5 Images about Bond Polarity | CK-12 Foundation like Carbon Dioxide Non Polar - Clarence-has-Pham, Is Carbon Dioxide (CO2) Polar Or Nonpolar? » Science ABC and also Bond Polarity | CK-12 Foundation. Here it is:
Bond Polarity | CK-12 Foundation
 www.ck12.orgpolarity carbon dioxide polar nonpolar bond formaldehyde why compound bonds
www.ck12.orgpolarity carbon dioxide polar nonpolar bond formaldehyde why compound bonds
Why Is Carbon Dioxide Polar Or Nonpolar ? Why Is Carbon Dioxide A Non
 invernessgangshow.netcarbon dioxide molecule carbone polar dioxyde nonpolar lewis dipole molécule chimicamo atome atomes miscela molecules polarity orbital hybridization linéaire mo
invernessgangshow.netcarbon dioxide molecule carbone polar dioxyde nonpolar lewis dipole molécule chimicamo atome atomes miscela molecules polarity orbital hybridization linéaire mo
Carbon Dioxide Non Polar - Clarence-has-Pham
 clarence-has-pham.blogspot.comIs Carbon Dioxide (CO2) Polar Or Nonpolar? » Science ABC
clarence-has-pham.blogspot.comIs Carbon Dioxide (CO2) Polar Or Nonpolar? » Science ABC
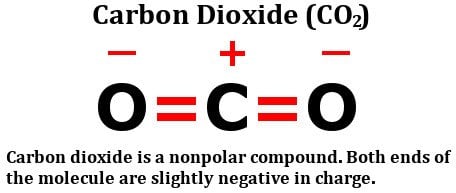 www.scienceabc.comcarbon dioxide co2 polar nonpolar structure bonds molecule bond look combined considered effect must let then these
www.scienceabc.comcarbon dioxide co2 polar nonpolar structure bonds molecule bond look combined considered effect must let then these
Is Carbon Dioxide (CO2) Polar Or Nonpolar? » Science ABC
 www.scienceabc.comcarbon dioxide polar co2 nonpolar science
www.scienceabc.comcarbon dioxide polar co2 nonpolar science
Carbon dioxide non polar. Carbon dioxide molecule carbone polar dioxyde nonpolar lewis dipole molécule chimicamo atome atomes miscela molecules polarity orbital hybridization linéaire mo. Is carbon dioxide (co2) polar or nonpolar? » science abc